Shedding of N-acetylglucosaminyltransferase-V is regulated by ... - Nature.com
Abstract
The number of N-glycan branches on glycoproteins is closely related to the development and aggravation of various diseases. Dysregulated formation of the branch produced by N-acetylglucosaminyltransferase-V (GnT-V, also called as MGAT5) promotes cancer growth and malignancy. However, it is largely unknown how the activity of GnT-V in cells is regulated. Here, we discover that the activity of GnT-V in cells is selectively upregulated by changing cellular N-glycans from mature to immature forms. Our glycomic analysis further shows that loss of terminal modifications of N-glycans resulted in an increase in the amount of the GnT-V-produced branch. Mechanistically, shedding (cleavage and extracellular secretion) of GnT-V mediated by signal peptide peptidase-like 3 (SPPL3) protease is greatly inhibited by blocking maturation of cellular N-glycans, resulting in an increased level of GnT-V protein in cells. Alteration of cellular N-glycans hardly impairs expression or localization of SPPL3; instead, SPPL3-mediated shedding of GnT-V is shown to be regulated by N-glycans on GnT-V, suggesting that the level of GnT-V cleavage is regulated by its own N-glycan structures. These findings shed light on a mechanism of secretion-based regulation of GnT-V activity.
Introduction
Protein functions are strictly regulated by post-translational modifications, including phosphorylation, methylation, lipidation, and glycosylation. Most proteins in the secretory pathway undergo glycosylation. Asparagine-linked glycosylation (N-glycosylation), a major type of glycosylation, is well conserved among various organisms and is essential for protein folding, trafficking, activity, and signal transduction1,2,3,4. Alterations in N-glycan structures can cause disease development and aggravation, for example in Alzheimer's disease, diabetes, and cancer5,6,7,8. Therefore, it is pivotal to understand how N-glycan biosynthesis is regulated in cells and how it is dysregulated in diseases.
Biosynthesis of N-glycans starts in the endoplasmic reticulum (ER), and a common precursor oligosaccharide consisting of 14 sugar residues is transferred en bloc to the Asn residue of an N–X–S/T consensus sequence (where X is any amino acid except Pro)3. N-Glycosylated proteins are then transported to the Golgi after trimming of three glucose (and one mannose) residues, and N-glycans are further modified by various glycosidases and glycosyltransferases. This process converts immature oligomannose-type N-glycans to the mature complex-type N-glycans, which have varying numbers of branches (Fig. 1a). N-Acetylglucosaminyltransferase (GnT)-III9, -IV10, and -V6,11 transfer GlcNAc to β-Man via a β1,4-linkage, to α1,3-Man via a β1,4-linkage, and to α1,6-Man via a β1,6-linkage, respectively (Fig. 1a). In addition to these GlcNAc branches, a fucose residue can be attached to the innermost GlcNAc residue by fucosyltransferase 8 (FUT8)6fucosyltransferase. J. Biol. Chem. 271, 27810–27817 (1996)." href="https://www.nature.com/articles/s42003-022-03697-y#ref-CR12" id="ref-link-section-d217055036e569">12,13 (Fig. 1a).
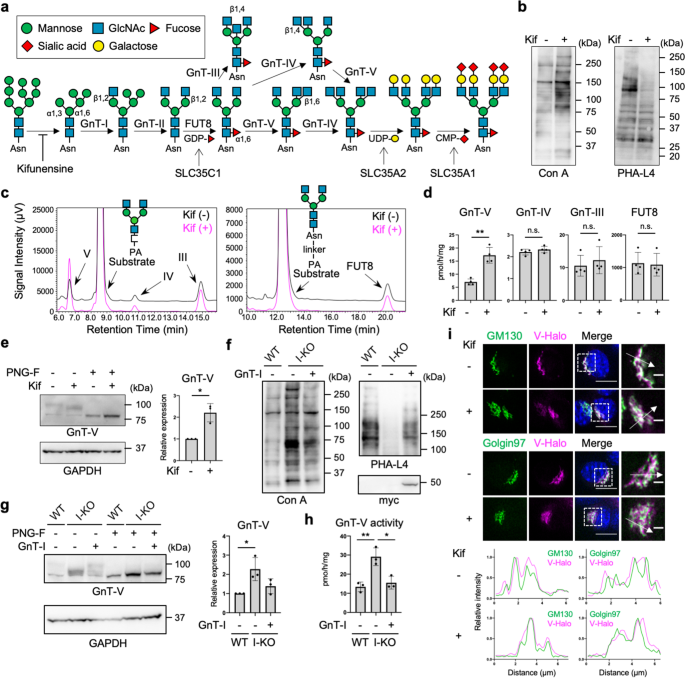
a Scheme of biosynthesis pathway of N-glycans. Glycan symbols are according to the standard symbol nomenclature for glycans93. Asn: asparagine. b Lectin blotting of proteins from HEK293 cells treated with or without kifunensine (kif). c In vitro enzyme assays of GnTs (left) and FUT8 (right) in HEK293 cells treated with or without kifunensine. For GnTs, GnGnbi-PA was used as an acceptor substrate. For FUT8, GnGnbi-Asn-PNS was used as an acceptor substrate. The product peak for each enzyme reaction was identified from control reactions using each purified recombinant enzyme, as shown in Supplementary Fig. 1. d Quantification of the activities of GnTs and FUT8 as measured in (c). Error bars represent SD (n = 4). Statistical analysis was by unpaired Student's t-test. e Proteins from HEK293 cells treated with or without kifunensine were blotted for GnT-V and GAPDH. Before SDS-PAGE, cell lysates were treated with or without PNGase F (PNG-F). The graph shows quantification of the GnT-V signals in western blots. Error bars represent SD (n = 3). Statistical analysis was by Welch's t-test. f Proteins from wild-type (WT), GnT-I-deficient (I-KO), and GnT-I (myc-tagged)-rescued HEK293 cells were blotted with Con A, PHA-L4, or anti-myc antibody. g Proteins from WT, GnT-I-deficient (I-KO), and GnT-I-rescued HEK293 cells were treated with or without PNGase F and blotted for GnT-V and GAPDH. The graph shows quantification of the GnT-V signals in PNGase F-treated blots. Error bars represent SD (n = 3). Statistical analysis was by one-way analysis of variance (ANOVA) with post-hoc Tukey test. h The cellular activity of GnT-V in WT, GnT-I-deficient (I-KO), and GnT-I-rescued HEK293 cells. Error bars represent SD (n = 3). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. i Localization of Halo-tagged GnT-V (V-Halo) visualized in B16 cells treated with or without kifunensine. (Upper) GM130 and Golgin97 were stained as cis- and trans-Golgi markers, respectively. (Lower) Line plots of relative fluorescence intensity are shown. Arrows indicate the plotted regions. Scale bars: 10 or 2 µm. Staining with Calnexin, an ER marker, was shown in Supplementary Fig. 5. *p < 0.05; **p < 0.005; n.s. not significant.
These branched structures of N-glycans have close relationships to specific diseases. For example, bisecting GlcNAc, which is the GlcNAc branch synthesized by GnT-III, is involved in Alzheimer's disease because the loss of GnT-III resulted in both the reduction of amyloid-β deposition and improvement of cognitive function in Alzheimer's disease mouse models, owing to the impaired function of amyloid-β-producing enzyme BACE114,15. Knockout (KO) mice of another GlcNAc-branching enzyme—GnT-IVa—exhibited a type 2 diabetes-like phenotype because of the impaired function of glucose transporter 2 in pancreatic β-cells16.The β1,6-GlcNAc branch synthesized by GnT-V is involved in cancer development and malignancy as tumor growth and metastasis were greatly inhibited in GnT-V-KO mice17. Mechanistically, galectin-3-mediated retention of epidermal growth factor receptor at the cell surface and downstream signaling were reduced in GnT-V-KO cells18. Moreover, knocking down GnT-V inhibited cell migration and invasion, probably because of the reduced β1,6-GlcNAc level on N-cadherin19. These observations indicate that regulation of the biosynthesis of N-glycan branches is a potential therapeutic approach to these diseases. Therefore, it is of critical importance to fully understand the regulatory mechanisms of the glycosyltransferases involved in N-glycan branching.
Several regulatory factors of glycosyltransferase activities have been reported, such as subcellular localization20,21,22,23 and formation of enzyme complexes24,25,26. These post-transcriptional mechanisms greatly impact glycosyltransferase activity and the resultant glycan profiles in cells. Furthermore, cellular activities of glycosyltransferases for N-glycan biosynthesis were shown to mutually affect each other by compensatory or feedback mechanisms. For example, loss of bisecting GlcNAc synthesized by GnT-III resulted in elevation of many terminal modifications of N-glycans such as galactosylation, sialylation, and human natural killer-1 modification27. In another study, loss of two GlcNAc branches (β1,2- and β1,6-linked GlcNAc residues on α1,6-Man) in N-glycans by knocking out GnT-II led to hyper-N-acetyllactosamine extension of the remaining GlcNAc branch for functional compensation in T cells28. Furthermore, FUT8-KO fibroblasts showed selective elevation of GnT-III activity29. These findings suggest that cells are equipped with regulatory systems for glycosylation activity to adapt to changes in cellular glycosylation.
Recently, proteolytic release of glycosyltransferase into extracellular space has also been found to regulate cellular activity of glycosyltransferases. Although the presence of cleaved soluble forms of glycosyltransferases in body fluids has been known for a long time30,31,32,33, its physiological significance remains elusive. Recently, signal peptide peptidase-like 3 (SPPL3) was identified as being responsible for proteolytic cleavage of various glycosyltransferases34,35,36. Among them, GnT-V is one of the best characterized SPPL3 substrates. It was demonstrated that the C-terminal region of the transmembrane domain of GnT-V is cleaved by SPPL3, resulting in secretion of the protein into the culture medium34. Knocking out SPPL3 significantly reduced secretion of GnT-V, thereby elevating the cellular levels of GnT-V protein and its product glycans34. This clearly demonstrated the importance of SPPL3-dependent shedding of GnT-V for cellular GnT-V activity. However, it has not been elucidated how this proteolytic cleavage and secretion of GnT-V by SPPL3 is regulated in cells.
In this study, we sought to unveil a novel regulatory mechanism of the activity of N-glycan branching enzymes in cells. We hypothesized that blockade of certain steps in N-glycan maturation in cells specifically induces an activity change of an enzyme for N-glycan branching through compensation or feedback mechanisms. We found that loss of complex-type N-glycans by knocking out various genes or using a glycosylation inhibitor commonly resulted in a specific increase in GnT-V activity. We also found that such activation of GnT-V is caused by impaired cleavage of GnT-V by SPPL3. Our findings highlight a novel regulatory strategy of cells to maintain glycosyltransferase activities.
Results
Loss of complex-type N-glycans specifically increased protein expression and activity of GnT-V
To explore the impact of altered N-glycosylation status on the activities of glycosyltransferases for N-glycan branching, we first treated human embryonic kidney (HEK) 293 cells with a mannosidase inhibitor—kifunensine—to inhibit the conversion of immature oligomannose-type N-glycans to the complex-type N-glycans (Fig. 1a). Conversion of cellular N-glycans by kifunensine treatment was confirmed by the increased reactivity of cellular proteins to mannose-reactive concanavalin A (Con A) lectin37 and decreased reactivity to leuko-agglutinating phytohemagglutinin (PHA-L4), which recognizes the GnT-V-produced branch38 (Fig. 1b and Supplementary Fig. 1). To examine the enzyme activities of GnT-III, -IV, -V, and FUT8, lysates of cells treated with or without kifunensine were incubated with their acceptor substrates, GnGnbi-PA (for GnTs) or GnGnbi-Asn-PNS (for FUT8), followed by separation of the products from the unreacted substrates by reverse-phase high-performance liquid chromatography (HPLC) (Fig. 1c), as established previously39,40. The retention times of the products of the enzyme reactions were confirmed by control reactions using purified recombinant enzymes (Supplementary Fig. 2a, b). We quantified the activities of these enzymes in the cell lysates by calculating the peak areas of the products, and found that the activities of GnT-III, -IV, and FUT8 in kifunensine-treated cells were almost the same as in untreated cells (Fig. 1c, d). In contrast, GnT-V activity in kifunensine-treated cells was approximately 2.5-times higher than that in untreated cells (Fig. 1c, d), suggesting that GnT-V activity is selectively upregulated by blocking N-glycan maturation. We next examined the expression levels of endogenous GnT-V protein by western blotting in which cell lysates were treated with N-glycosidase F (PNGase F) to see the immunoreactive band of GnT-V more clearly by removing N-glycans. We found that the level of GnT-V protein in cells was also increased by kifunensine treatment (Fig. 1e), indicating that the loss of complex-type N-glycans upregulated GnT-V activity by enhancing the amount of protein in cells.
To examine whether these phenomena were also observable in other cell types, we conducted the same experiments in Neuro-2A cells (a mouse neuroblastoma cell line). The activity and the level of GnT-V protein were both increased by kifunensine treatment (Supplementary Fig. 3a, b). To further examine whether blockade of biosynthesis of complex-type N-glycans generally upregulates GnT-V activity in cells, we examined the cellular GnT-V activity in GnT-I-deficient HEK293S cells, from which complex-type N-glycans are completely lost41 (Fig. 1a, f). Both the activity and the level of GnT-V protein were increased in GnT-I-KO cells compared with wild-type (WT) HEK293 cells, to similar levels as in kifunensine-treated HEK293 cells (Fig. 1g, h). Transfection of a GnT-I-myc-tag construct into GnT-I-KO cells cancelled the increased activity and the level of GnT-V protein (Fig. 1g, h), confirming the requirement of GnT-I for regulating the level of GnT-V protein in cells. To further confirm the effects of GnT-I-KO on the level of GnT-V protein, we performed the same experiments in the GnT-I-deficient Chinese hamster ovary (CHO) cell line Lec142,43 (Supplementary Fig. 3c). As expected, both activity and the level of GnT-V protein were increased in Lec1 cells compared with WT CHO cells, which was reversed by GnT-I overexpression (Supplementary Fig. 3d, e).
To investigate whether N-glycan maturation states also affect GnT-V localization, we compared its localization between control and kifunensine-treated cells. For this purpose, we stably expressed human GnT-V fused with a Halo7 tag (GnT-V-Halo) in B16 cells (a murine melanoma cell line), and we confirmed by in vitro enzyme assays that GnT-V-Halo retained enzyme activity (Supplementary Fig. 4a, b), excluding that addition of the Halo-tag inhibited GnT-V functions. We used B16 cells for these experiments, because HEK293 cells were easily detached during staining. Co-staining with cis- and trans-Golgi markers GM130 and Golgin97 indicated that GnT-V-Halo signals were well overlapped with both GM130 and Golgin97 signals, particularly with GM130, in cells with or without kifunensine treatment (Fig. 1i). On the other hand, co-staining with an ER marker Calnexin showed that the pattern of GnT-V-Halo signals was clearly different from that of Calnexin with or without kifunensine treatment (Supplementary Fig. 5), suggesting that kifunensine-treatment hardly altered the localization of GnT-V-Halo. Taken together, these results demonstrate that loss of complex-type N-glycans specifically increased the protein level and activity of GnT-V without affecting its subcellular localization.
Secretion of GnT-V by SPPL3-dependent proteolysis is inhibited by blocking N-glycan maturation
Next, we attempted to elucidate the mechanism of how the level of GnT-V protein was elevated. We first examined the mRNA levels of GnT-V in GnT-I-deficient HEK293S cells. The levels of GnT-V mRNA (encoded by the MGAT5 gene) were found to be comparable between WT and GnT-I-deficient cells (Fig. 2a), indicating that the level of GnT-V protein is post-transcriptionally upregulated in GnT-I-KO cells. Considering that GnT-I is a Golgi-resident glycosyltransferase and functions in the post-ER organelles, it is less likely that loss of GnT-I affected the general translational regulation machinery. Therefore, we hypothesized that the level of GnT-V protein is post-translationally upregulated by loss of complex-type N-glycans. To further investigate the mechanism, we next focused on degradation of GnT-V protein. HEK293 cells were treated with MG132 (a proteasomal inhibitor) or chloroquine (a lysosomal inhibitor) for 24 h, and the levels of GnT-V protein were examined. While β-catenin and p62, which are subjected to degradation in proteasomes and lysosomes, respectively44,45, were accumulated in cells treated with these compounds, neither the level of GnT-V protein nor activity in cells was increased by treatment with either compound (Fig. 2b, c). This suggests that degradation of GnT-V protein has at most a minor role in regulation of the level of GnT-V protein. Instead, it was recently reported that GnT-V undergoes proteolytic cleavage by SPPL3 and is subsequently shed into the extracellular space34. Thus, we investigated whether the secretion of GnT-V is inhibited in GnT-I-deficient cells. We found that the level of secreted GnT-V in the culture medium of GnT-I-deficient cells was greatly decreased compared with WT HEK293 cells (Fig. 2d), strongly suggesting that shedding of GnT-V is inhibited by the loss of N-glycan maturation. We next established an SPPL3-KO cell line; the Sppl3 gene was knocked out from B16 cells using the CRISPR-Cas9 system. We used B16 cells here, because endogenous GnT-V proteins is more easily detected in B16 than in HEK293 cells. The genotype of the SPPL3-KO clone was confirmed by PCR and genome sequencing (Supplementary Fig. 6a, b). Consistent with previous study34, knocking out SPPL3 resulted in both abolition of GnT-V secretion and concomitant accumulation of GnT-V protein in cells (Fig. 2e, top row of the left-hand panel), confirming that SPPL3 is the dominant enzyme for GnT-V shedding. Kifunensine treatment of WT cells, which resulted in increased Con A signals and decreased PHA-L4 signals (Fig. 2e, middle and right-hand panels), again significantly increased the level of GnT-V protein and activity in cells (Fig. 2e left-hand panel, first and second lanes, and Fig. 2f), while it decreased the levels of secreted GnT-V (Fig. 2e left-hand panel, fifth and sixth lanes). In sharp contrast, in SPPL3-KO cells, the levels of GnT-V protein (Fig. 2e left-hand panel, third and fourth lanes) and activity (Fig. 2f) in cells were no longer increased by kifunensine treatment. This demonstrates that blockade of N-glycan maturation inhibits SPPL3-dependent shedding of GnT-V, leading to the increased levels of GnT-V protein and activity in cells.
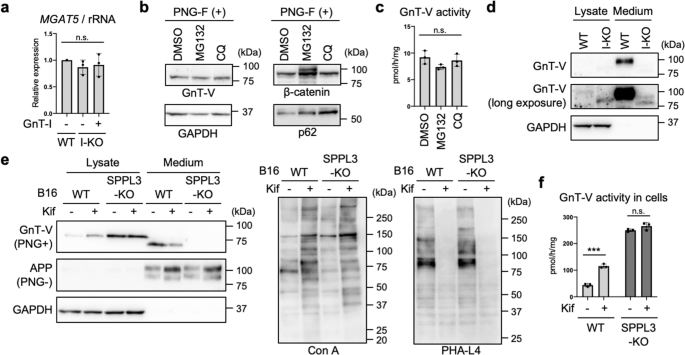
a Relative mRNA expression of MGAT5 (encoding GnT-V) in WT, GnT-I-deficient (I-KO), and GnT-I-rescued HEK293 cells. The levels of MGAT5 mRNA were normalized by those of rRNA. Error bars represent SD (n = 3). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. b Proteins from HEK293 cells treated with dimethylsulfoxide (DMSO), MG132, or chloroquine (CQ) were blotted for GnT-V, β-catenin, p62, or GAPDH. The samples were treated with PNGase F (PNG-F) before SDS-PAGE. c The activity of GnT-V in DMSO-, MG132-, or CQ-treated HEK293 cells. Error bars represent SD (n = 3). Statistical analysis was by one-way ANOVA with post-hoc Dunnett test. d Proteins in the lysates of WT and GnT-I-deficient cells and in the culture media of these cells were blotted for GnT-V or GAPDH. e WT and SPPL3-deficient B16 cells were treated with or without kifunensine (kif). Proteins in the cell lysates and the culture media of these cells were blotted for GnT-V, APP, or GAPDH. The samples were treated with or without PNGase F before SDS-PAGE. APP was blotted as a positive control of secreted protein94. The proteins in the cell lysates were also blotted with Con A or PHA-L4. f The cellular activity of GnT-V in WT and SPPL3-KO B16 cells treated with or without kifunensine. Error bars represent SD (n = 3). Statistical analysis was by two-way ANOVA with post-hoc Sidak test. ***p < 0.0005; n.s. not significant.
Impact of loss of complex-type N-glycans on the expression, localization, and activity of SPPL3
We next investigated the mechanism of how cellular N-glycan states regulate SPPL3-dependent shedding of GnT-V. We first observed that the level of SPPL3 mRNA in GnT-I-deficient HEK293S cells was comparable to that in WT or rescued cells (Fig. 3a), excluding that the decreased GnT-V shedding was caused by downregulation of SPPL3 gene expression. On the basis of these findings, we reasoned that N-glycans attached to either SPPL3, GnT-V, or their regulator proteins, affect the broad or GnT-V-specific cleavage activity of SPPL3.
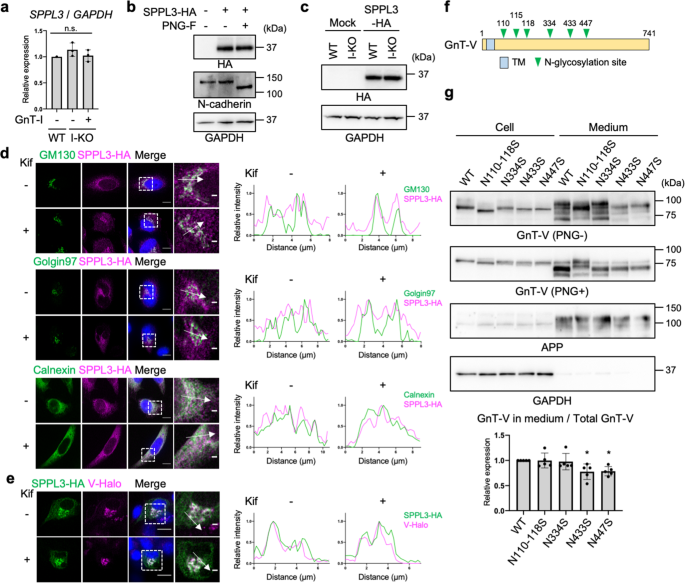
a Relative mRNA expression of SPPL3 in WT, GnT-I-deficient (I-KO), and GnT-I-rescued HEK293 cells. The levels of SPPL3 mRNA were normalized by those of GAPDH. Error bars represent SD (n = 3). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. b SPPL3-3HA was expressed in HEK293 cells, and the lysates were treated with or without PNGase F (PNG-F). The proteins in the lysates were blotted for HA, N-cadherin, or GAPDH. c SPPL3-3HA was expressed in WT or GnT-I-deficient cells and the cell lysates were blotted for HA or GAPDH. d (Left) HeLa cells expressing SPPL3-3HA by pTK plasmid were treated with or without kifunensine and immunostained for HA and GM130, Golgin97, or Calnexin. (Right) Line plots of relative fluorescence intensity are shown. Arrows indicate the plotted regions. Scale bars: 10 or 2 µm. e (Left) B16 cells stably expressing GnT-V-Halo was transfected with SPPL3-3HA and were treated with or without kifunensine. SPPL3-3HA was immunostained with anti-HA antibody and GnT-V-Halo was visualized by TMR. (Right) Line plots of relative fluorescence intensity are shown. Arrows indicate the plotted regions. Scale bars: 10 or 2 µm. f Domain structure of human GnT-V. TM: Transmembrane domain. g GnT-V WT or its N-glycosylation site mutants were expressed in HEK293 cells. Proteins in the cell lysates and culture media of these cells were blotted for GnT-V, APP, or GAPDH. The samples were treated with or without PNGase F before SDS-PAGE. The graph shows quantification of the signal ratio of secreted GnT-V to total (cell + medium) GnT-V in PNGase F-treated blot. APP was blotted as a positive control of secreted protein. Error bars represent SD (n = 5). Statistical analysis was by one-way ANOVA with post-hoc Dunnett test. *p < 0.05; n.s. not significant.
It was previously reported that SPPL3 is not N-glycosylated despite the presence of potential N-glycosylation sites46. To confirm this, C-terminally 3 × human influenza hemagglutinin (3HA)-tagged human SPPL3 was expressed in HEK293 cells and detected by western blotting after treatment with or without PNGase F. SPPL3-3HA was detected as a single band in the untreated sample, and the band was not shifted by PNGase F treatment, strongly suggesting that no N-glycans are attached to SPPL3 (Fig. 3b). We next investigated whether an N-glycosylated protein other than SPPL3 itself affects expression or localization of SPPL3 in an N-glycan structure-dependent manner. Recently, it was reported that expression of SPPL3 protein is regulated by the glycoprotein SPPL2c47, but we found that the levels of SPPL3-3HA protein were not decreased in GnT-I-deficient HEK293S cells (Fig. 3c). Although translational regulation of SPPL3 mRNA through 5'- or 3'- untranslated regions was not examined in our expression system, it is less likely that knocking out the Golgi enzyme GnT-I directly affects translation of SPPL3 in the ER, because knocking out GnT-I likely affects biological processes within the Golgi or in post-Golgi compartments. Moreover, we observed that the localization of SPPL3-3HA was not altered by kifunensine treatment, the protein being mainly localized to the ER and the Golgi (as also previously reported48) regardless of the treatment (Fig. 3d), suggesting that SPPL3 encounters GnT-V in kifunensine-treated cells. As expected, co-localization of SPPL3-3HA and GnT-V-Halo was observed in both kifunensine-treated and -untreated cells (Fig. 3e). These results demonstrate that altered N-glycan structures had a negligible effect on the expression and localization of SPPL3.
Next, we investigated whether the activity of SPPL3 was affected by cellular N-glycan states. Because SPPL3 has no N-glycan, we hypothesized that N-glycan structures on GnT-V affect its proteolytic cleavage by SPPL3. To test this hypothesis, we mutated the consensus N-glycosylation sites on GnT-V and examined the secretion levels of the mutants. GnT-V has six N-glycosylation sites (Fig. 3f), and we deleted these sites by substituting Asn residues to Ser. These mutants exhibited GnT-V activity similarly to that of the WT (Supplementary Fig. 7), suggesting that they mostly maintained proper folding. N110-118S and N334S mutants were secreted at similar levels to WT GnT-V, whereas secretion of N433S and N447S mutants was decreased to approximately 80% of that of the WT (Fig. 3g) (note that N110-N118S is a mutant in which residues N110, N115, and N118 are all mutated to serine). Together, these results suggest that specific N-glycans on GnT-V regulate its shedding by SPPL3.
Terminal modifications but not branching of N-glycans regulate GnT-V shedding
Kifunensine treatment or GnT-I KO resulted in loss of all complex-type N-glycans, and therefore we next examined the detailed N-glycan structures required for the SPPL3-dependent secretion of GnT-V. We first focused on the terminal structures, including fucose, galactose, and sialic acid (Fig. 1a). To investigate the role of fucosylation, we examined the activity of GnT-V in fucosylation-deficient cells. For this purpose, SLC35C1, which encodes a GDP-fucose transporter49, was knocked out from HEK293 cells using the CRISPR-Cas9 system. Genotyping PCR (Supplementary Fig. 8a) and blotting with Aleuria aurantia lectin (AAL) (Fig. 4a and Supplementary Fig. 1), which specifically binds to fucosylated glycans50, demonstrated the loss of fucosylation in SLC35C1-KO cells. We examined cellular GnT-V activity and the secretion of GnT-V, and found that GnT-V activity and the secretion of GnT-V in SLC35C1-KO cells were comparable to those in WT cells (Fig. 4b, c), indicating that fucosylation has no effect on GnT-V shedding. We next examined a previously established galactosylation-defective HEK293 cell line lacking SLC35A2, which encodes a UDP-galactose transporter51,52. To validate the glycan changes in SLC35A2-KO cells, we investigated the reactivity of the specific lectins, Griffonia simplicifolia lectin II (GSL-II) and Helix pomation lectin (HPA), which bind to terminal GlcNAc and N-acetylgalactosamine (GalNAc), respectively (Supplementary Fig. 1)53,54. Flow cytometry of SLC35A2-KO cells with GSL-II and HPA confirmed the loss of galactosylation, and transfection of human SLC35A2 gene restored the glycan profiles (Supplementary Fig. 8b). Blotting with galactose-specific Ricinus communis agglutinin I (RCA-I)55 also evidenced the loss of galactosylation from SLC35A2-KO cells (Fig. 4d, left-hand panel). We found that the protein level and activity of GnT-V were both higher in these cells than in WT cells (Fig. 4d middle and right-hand panels, and Fig. 4e), indicating that loss of galactosylation inhibited GnT-V shedding. To investigate the role of sialylation, we generated a sialylation-defective cell line lacking SLC35A1, which encodes a CMP-sialic acid transporter56,57, using the CRISPR-Cas9 system. Genetic deletion was validated by PCR (Supplementary Fig. 8c). Flow cytometry with Sambucus nigra lectin (SNA) and peanut agglutinin (PNA), which preferentially bind to α2,6-linked sialic acid and to O-GalNAc glycans with terminally exposed galactose, respectively (Supplementary Fig. 1)58,59, showed the loss of sialylation in the SLC35A1-KO cell line (Supplementary Fig. 8d). The increased signals from SLC35A1-KO cells in RCA-I blotting also confirmed the loss of sialylation (Fig. 4f left-hand panel). Similar to SLC35A2-KO cells, both the activity and level of GnT-V protein were higher in SLC35A1-KO cells than in WT cells (Fig. 4f middle and right-hand panels, and Fig. 4g), indicating that loss of sialylation inhibited GnT-V shedding. Transfection of the relevant genes into each KO cell line restored the altered levels of GnT-V protein and activity (Fig. 4d–g), ruling out off-target effects. Furthermore, GnT-V activity in culture medium was robustly decreased in SLC35A1-KO and SLC35A2-KO cells than in WT cells (Fig. 4h). Because sialic acids are mostly attached to galactose residues and they were depleted in both SLC35A1- and SLC35A2-KO cells, these results strongly suggest that loss of sialylation suppressed GnT-V shedding.
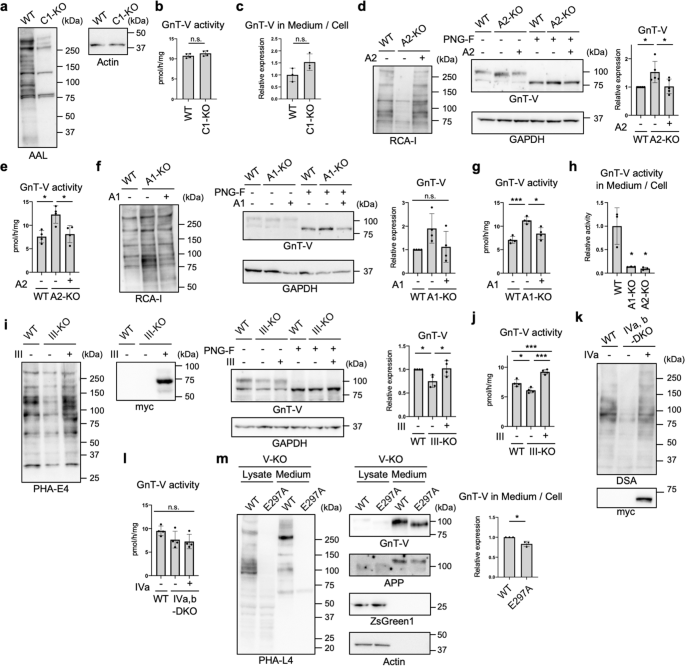
a Proteins from WT and SLC35C1-KO (C1-KO) HEK293 cells were blotted with AAL or anti-actin antibody. b The activity of GnT-V in WT and SLC35C1-KO HEK293 cells. Error bars represent SD (n = 4). Statistical analysis was by unpaired Student's t-test. c C-terminally 3 × FLAG-tagged GnT-V WT was expressed in WT and SLC35C1-KO HEK293 cells, and secretion of GnT-V in these cells were assessed by quantify the signal ratio of secreted GnT-V (in medium) to cellular GnT-V. Error bars represent SD (n = 3). Statistical analysis was by unpaired Student's t-test. d Proteins from WT, SLC35A2-KO (A2-KO), and SLC35A2-rescued HEK293 cells treated with or without PNGase F were blotted with RCA-I, and anti-GnT-V and anti-GAPDH antibodies. The graph shows quantification of the GnT-V signals in PNGase F-treated blots. Error bars represent SD (n = 5). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. e The cellular activity of GnT-V. Error bars represent SD (n = 4). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. f Proteins from WT, SLC35A1-KO (A1-KO), and SLC35A1-rescued HEK293 cells treated with or without PNGase F were blotted with RCA-I, and anti-GnT-V and anti-GAPDH antibodies. The graph shows quantification of the GnT-V signals in PNGase F-treated blots. Error bars represent SD (n = 4). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. g The cellular activity of GnT-V. Error bars represent SD (n = 4). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. h Cellular and secreted GnT-V activity from WT, A1-KO and A2-KO HEK293 cells were examined in vitro. The graph shows the relative activity of the ratio of secreted GnT-V (in medium) to cellular GnT-V. Error bars represent SD (n = 3). Statistical analysis was by one-way ANOVA with post-hoc Dunnett test. i Proteins from WT, GnT-III-KO (III-KO), and GnT-III (myc-tagged)-rescued HEK293 cells treated with or without PNGase F were blotted with PHA-E4, and anti-myc, anti-GnT-V, and anti-GAPDH antibodies. The graph shows quantification of the GnT-V signals in PNGase F-treated blots. Error bars represent SD (n = 4). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. j The cellular activity of GnT-V. Error bars represent SD (n = 4). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. k Proteins from WT, GnT-IVa and IVb-DKO (IVa,b-DKO), and GnT-IVa (myc-tagged)-rescued HEK293 cells were blotted with DSA and anti-myc antibody. l The cellular activity of GnT-V. Error bars represent SD (n = 4). Statistical analysis was by one-way ANOVA with post-hoc Tukey test. m GnT-V WT or its catalytically inactive E297A mutant was expressed in GnT-V-KO (V-KO) HEK293 cells. Proteins in the cell lysates and the culture media of these cells were blotted with PHA-L4, and anti-GnT-V, anti-APP, anti-ZsGreen1, or anti-actin antibodies. The graph shows quantification of the signal ratio of secreted GnT-V to cellular GnT-V. APP was blotted as a positive control of secreted protein. Error bars represent SD (n = 3). Statistical analysis was by Welch's t-test. *p < 0.05; ***p < 0.0005; n.s. not significant.
We also examined whether the number of GlcNAc branches in N-glycans affects the SPPL3-dependent secretion of GnT-V. To this end, the responsible enzymes—GnT-III (MGAT3) and -IV (MGAT4A and B)—were knocked out from HEK293 cells, respectively, using the CRISPR-Cas9 system. The recently generated GnT-V (MGAT5)-KO HEK293 cell line60 was also used. The genotype and loss of enzyme activity of the GnT-III-KO cell line was validated by PCR (Supplementary Fig. 9a), in vitro enzyme assays (Supplementary Fig. 9b), and lectin blotting (Fig. 4i, left-hand panel). In GnT-III-KO cells, the level of GnT-V protein and activity were not increased compared with that in WT cells (Fig. 4i, j), indicating that bisecting GlcNAc produced by GnT-III has only minor impacts on secretion and cellular expression of GnT-V. Considering GnT-IV, we knocked out both the GnT-IVa and -IVb isoenzymes61 from HEK293 cells (Supplementary Fig. 9c), and loss of the β1,4-GlcNAc branch from KO cells was confirmed by lectin blotting and flow cytometry using Datura stramonium agglutinin (DSA)62,63 (Fig. 4k and Supplementary Fig. 9d). In GnT-IVa and -IVb double-KO (DKO) cells, the activity of GnT-V was not increased compared with WT cells (Fig. 4l), suggesting that the β1,4-GlcNAc branch is not involved in GnT-V secretion. To next investigate the effects of the β1,6-GlcNAc branch, which is produced by GnT-V itself, on its secretion, we re-expressed WT GnT-V and a catalytically inactive form of GnT-V (E297A) in GnT-V-KO cells and examined the secretion. We and others recently reported crystal structures of GnT-V and revealed that mutation of the catalytic residue E297 resulted in complete loss of activity64,65. Consistent with this, expression of the E297A mutant did not restore PHA-L4 reactivity in GnT-V-KO cells (Fig. 4m left-hand panel). We observed that secretion of E297A mutant was only slightly decreased compared with the WT enzyme (Fig. 4m). Similar levels of ZsGreen1 (whose cDNA was inserted downstream of MGAT5-IRES2 sequence) in WT and E297A-expressing cells confirmed the similar transfection efficiencies of the WT- and E297A mutant-encoding plasmids (Fig. 4m). These data indicated that the β1,6-GlcNAc branch has only minor effects on GnT-V secretion. Collectively, our findings suggest that terminal galactosylation and sialylation, but not GlcNAc branching, enhance GnT-V shedding.
Enhanced GnT-V activity increases N-glycan branching in cells
To investigate whether the enhanced cellular activity of GnT-V resulted in increased GlcNAc branching of N-glycans in cells, we performed N-glycomic analysis. To this end, we compared N-glycan structures between WT cells and SLC35A2-KO cells in which cellular GnT-V activity was upregulated (Fig. 4d). N-Glycans were released from these cells, labeled with aminoxyTMT6 reagent, desialylated, and analyzed by liquid-chromatography–electrospray ionization-mass spectrometry (LC-ESI-MS). Glycomic data demonstrated that the overall N-glycan profile of SLC35A2-KO (A2-KO) cells was totally different from that in WT and rescued (A2KO + SLC35A2) cells, which mainly resulted from the loss of galactosylation in the SLC35A2-KO cells (Supplementary Fig. 10a). To more directly compare relative amounts of each number of GlcNAc branches, we simplified the glycan structures by removing galactose using β-galactosidase (Fig. 5a). Relative amounts of the complex-type glycans classified by the number of N-acetylhexosamine (HexNAc) units in antennas clearly showed that the less branched structures (having two or three HexNAcs) were decreased in KO cells, whereas the more highly branched structures (having four or five HexNAcs) were increased in KO cells (Figs. 5b, S10b, and Supplementary Data 2). By comparing with enzymatically prepared standard glycans, we further identified the GnT-V products among the glycans with three and four HexNAc residues (Supplementary Fig. 10c), and found that the levels of GnT-V products having four or five HexNAcs were drastically increased in KO cells (Fig. 5c). This demonstrates that the GnT-V activity enhanced in cells by blocking N-glycan maturation resulted in an increase in its product glycans.
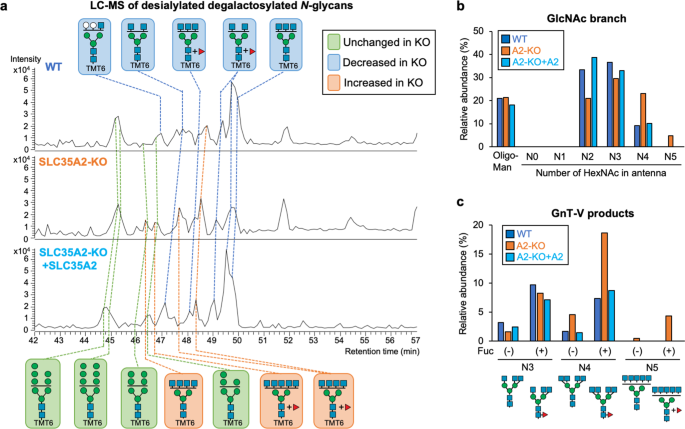
a Base peak chromatograms (BPCs) from LC-ESI MS analysis of TMT6-labeled desialylated β-galactosidase-treated N-glycans from WT, SLC35A2-KO (A2-KO), and SLC35A2-rescued (A2-KO + A2) HEK293 cells. The deduced structures of the major N-glycans are shown. Orange, glycans that increased in A2-KO cells compared with in WT cells; blue, glycans that decreased in A2-KO cells compared with in WT cells; green, glycans unchanged between WT and A2-KO cells. b The signal intensities of oligomannose glycans, and complex-type glycans with 0, 1, 2, 3, 4, and 5 HexNAc residues (HexNAc in chitobiose is excluded) from LC-MS analysis. c The signal intensities of GnT-V product glycans with 3, 4, and 5 HexNAc residues (HexNAc in chitobiose is excluded) with or without a Fuc residue from LC-MS analysis.
Glycan-dependent regulation of SPPL3-mediated shedding is substrate-selective
Finally, we investigated whether secretion of SPPL3 substrates other than GnT-V is also regulated by cellular N-glycan structures. Previously, CANT1 and B4GALT1, both of which are type-II transmembrane proteins involved in glycosylation66,67,68, were reported to be secreted in an SPPL3-dependent manner34,35. Consistent with this, we observed that secretion of these enzymes was almost completely inhibited in SPPL3-KO HEK293 cells (Fig. 6a) which were generated by CRISPR-Cas9 (Supplementary Fig. 6c). We then investigated whether secretion of these enzymes was also suppressed in GnT-I-deficient cells. In contrast to the severe inhibition of GnT-V shedding in such conditions, secretion of CANT1 was partially inhibited in GnT-I-KO cells, and B4GALT1 secretion was barely affected (Fig. 6b). These results suggest that regulation of SPPL3-mediated shedding is substrate-dependent, and that shedding of multiple glycan-related enzymes, including GnT-V, are selectively regulated by cellular N-glycan structures.
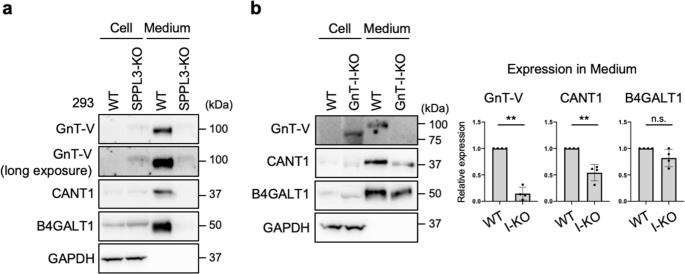
a Proteins in the lysates of WT and SPPL3-deficient HEK293 cells and in the culture medium of these cells were blotted for GnT-V, CANT1, B4GALT1, or GAPDH. b Proteins in the lysates of WT and GnT-I-deficient (I-KO) HEK293 cells and in the culture medium of these cells were blotted for GnT-V, CANT1, B4GALT1, or GAPDH. The graphs show quantification of the signal intensities of GnT-V, CANT1, and B4GALT1 secreted from I-KO cells relative to secretion from WT cells. Error bars represent SD (n = 4). Statistical analysis was by Welch's t-test. **p < 0.005; n.s. not significant.
Comments
Post a Comment